Singapore HSA has published the finalised Guidance on the Medical Device Unique Device Identification (UDI) System on 27 August 2021.
This guidance document is intended to provide clarity on the regulatory requirements for Unique Device Identification (UDI) implementation in Singapore and the details on the steps to submit UDI information into the Singapore Medical Device Register (SMDR) and Class A Medical Device Database.
In line with the internationally harmonised principles published by the International Medical Device Regulators Forum (IMDRF), the UDI system will comprise of:
- Development of unique device identifiers (UDIs) based on globally harmonised standards.
- Placement of UDIs in human readable interpretation (HRI) and Automated Identification for Data Capture (AIDC) formats on device package labels of the smallest unit of supply and on all higher levels of packaging or in some cases directly marked on the devices.
Note: UDIs applied on the medical device labels for EU or the USA markets will be accepted as is for Singapore.
Submission of minimum additional necessary UDI data elements such as UDI-DI to UDI Databases (UDID) by registrants, local manufacturers and importers. In the case of Singapore, the UDIDs will be the Singapore Medical Device Register (SMDR) for medical devices with risk Class B or higher and Class A Medical Devices database for Class A medical devices.
Manufacturers or Product owners are responsible for accurately assigning and placing the UDI in HRI and AIDC formats on the device label or on the device itself and on all higher levels of device package level hierarchy following the issuing agency’s specifications.
Medical Devices marketed in the USA and/or EU:
- Manufacturer or Product owners whose medical devices are marketed in the USA and/or EU and have been labelled with UDI based on the US or EU requirements can use these UDI as is for Singapore. Registrants of registered Class B, C and D medical devices or importer of listed Class A medical devices can submit the UDI information as is to the SMDR or Class A Medical Device Database.
- Medical Devices not marketed in the USA or EU:
- Manufacturers or product owners whose medical devices are not marketed in the USA or EU, are required to develop and implement UDI for Singapore. They should choose an issuing agency designated by HSA for implementing the UDI system and assign UDI to their medical devices based on the requirements specified in this guidance document.
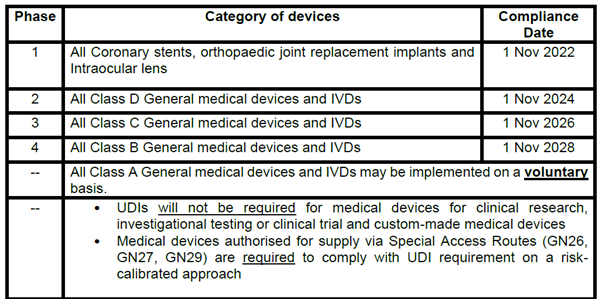
To allow for adequate preparation time for all stakeholders, the requirement for medical devices to be labelled with UDI prior to their placement on Singapore market (i.e. including those supplied via Special Access Routes) will be implemented in phases based on a risk-calibrated approach. (see table below for compliance date according to category of device)
Medical devices that are supplied in Singapore after the respective compliance date based on the risk class, are required to comply with UDI requirement unless otherwise specified.
Note: The compliance date for the various phases is tentative and is subject to adjustments based on the progress of the earlier phases of implementation.
All medical devices imported into Singapore must be labelled with UDI from the respective UDI compliance dates. However, companies will be given additional 6 months from the compliance date to deplete the respective medical devices that have been imported prior to the compliance date and exist in their current supply chain.
For more details, please refer to
Guidance on Medical Device UDI System, August 2021: https://www-hsa-gov-sg-admin.cwp.sg/docs/default-source/hprg-mdb/gudiance-documents-for-medical-devices/guidance-on-medical-device-udi-system-(27aug_pub).pdf
FAQ (Medical Device UDI System), August 2021: https://www.hsa.gov.sg/docs/default-source/hprg-mdb/gudiance-documents-for-medical-devices/faq-(medical-device-udi-system)-2021.pdf