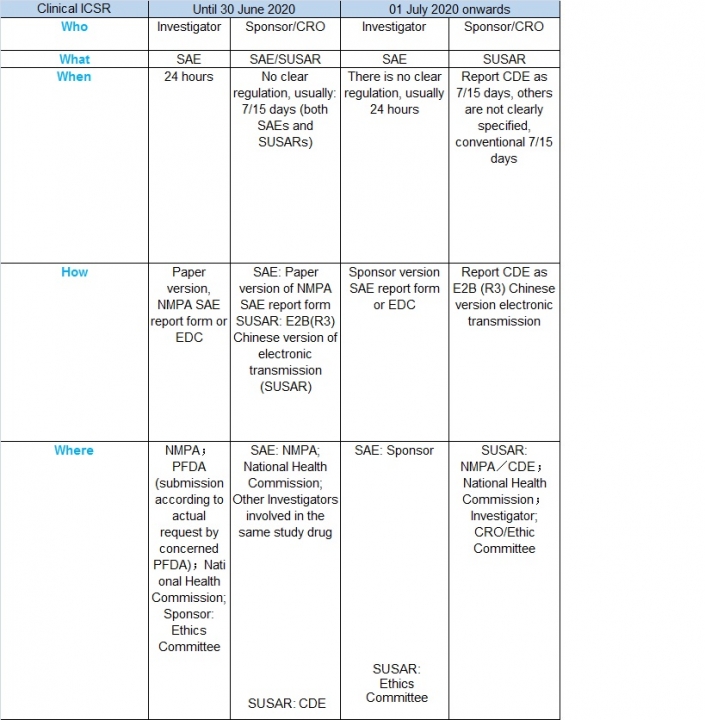
In order to deepen the reform of the drug review and approval system, encourage innovation, and further promote the research and improvement of the quality of drug clinical trials in China, the State Drug Administration, and the National Health and Health Commission organized and revised the “Clinical Drug Quality Control Standards”, which is released on April 23 2020 and effective from July 1, 2020.
The Chinese standards are essentially a GCP document which has been harmonized with ICH standards.
The document provides clarification of sponsor and investigator reporting responsibilities for clinical safety reports.
For more information, please refer to: http://www.nmpa.gov.cn/WS04/CL2138/376852.html
